These are more experimental observations, all intensely interesting. The conservation of total energy and momentum holds without reference to fluorescence at all, so will the important photon mass calculation. The fact that frequency shifts are seen in a non absorbing martial means that they are not fluorescence. So now I will proceed to writing up Sections 1 and 2 of UFT279, a four author paper with Horst Eckardt, Gareth Evans and Trevor Morris, the two experimentalists. As GJE mention, anyone can easily reproduce these effects in glass with a laser and at home.
Sent: 30/11/2014 09:47:35 GMT Standard Time
Subj: Re: Fwd: Re: Question on fluorescence /absorption at 536nmThanks both. I have had a quick look at this paper (could one of you send Myron a copy because I have broken the link somehow and now lost the paper at my end).
I agree with the general conclusion about the potential use of olive oil in laser technology. Their experiment is almost a perfect replica of Robert Fosbury ‘ s work (or vice-versa). It is good that there are now more examples of confirmation of the actual observations emerging.
The authors of this paper (and Robert) attribute the frequency shifts to the chlorophyll at 680nm to fluoresence (after all this is all that was known to them).
There are indeed some relatively weak absorptions in their transmission spectrum of olive oil. The authors of this paper “use these absorptions to excite the chlorophyll molecules and create the fluorescence”.
However, these weak absorptions are probably not chlorophyll absorptions. Olive oil does not just contain chlorophyll. These weak absorptions are almost certainly associated with another component in olive oil. A spectrum of neat chlorophyll can be seen in the link below:
http://www.chm.bris.ac.uk/motm/chlorophyll/chlorophyll_h.htm
There are no absorptions at all in the green part of the visible spectrum (which is why I have focused on it). So, they are not exciting chlorophyll molecules with green laser light (but some other molecules – if any at all). If they are not exciting chlorophyll molecules the incident radiation is not causing chlorophyll molecules to fluoresce. But their frequency shifts are to the well known chlorophyll absorption at around 680nm. This cannot be a consequence of chlorophyll fluorescence. They have not looked closely enough at the source of their weak absorption lines.
Also, remember that olive oil frequency shifts white light itself to the chlorophyll absorption at 680nm (photo 4). That is, all the individual frequencies in this broad band source are shifted to the same frequency. If they had chosen laser light well away from their weak absorptions they would still have observed a frequency shift to the same chlorophyll absorption
If we can get close to reproducing these frequency shifts in olive oil (or formaldehyde, or both) it is going to cast great doubt over the interpretaion of much of the fluorescent work published in the literature. We now have a much better explanation of frequency shifts based on accurate first principles. Optics as we have known it was wrong. There are always frequency shifts in absorption and reflection.
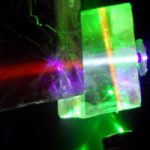
As for calcite Horst, if you can get hold of some Icelandic Spar you will easily see the frequency shifts. This crystal was used by Newton, and others around the same time, in discoveries that effectively laid the foundations of most of classic optics as we know it. They missed some things though because they did not have lasers (see photo 1). The first Icelandic Spar crystal on the right in the photo is illuminated by blue and green laser light at the same time from different directions. Green laser light changes to yellow and blue laser light to pale blue / white light in the first crystal. Blue laser light changes to red in the second crystal (pink Icelandic Spar). Fluorescence?
The best way to argue againgst fluorecence as we thought of it though was to find frequency shifts in glass itself. We have already reported a frequency shift in a glass prism using blue / violet laser light but knew we needed to find a shift with green laser light (glass strongly absorbs at the uv end of the spectrum so the farther we can see shifts away from that end of the spectrum with visible light the better). We have had last seen a pronounced shift in glass with green laser light (photos 2 and 3). There is no absorption in glass at this frequency (see the clear glass spectrum in the link below):
http://www.shimadzu.com/an/industry/ceramicsmetalsmining/chem0501005.htm
Fluorescence?
All the best, Gareth
Sent from Samsung Mobile
- sdc14995b
- 20141130 084810
- 20141130 084708
- 20141028 2337551